We use a broad range of complementary techniques to capture the structure and dynamics of large molecular assemblies. Specifically, we focus on the dynamic molecular interaction between DNA, the spindle, the lamina, and the membrane. Our approaches include structural biology, biochemical assays and reconstitutions, biophysics, proteomics, cell biology and AI-guided predictions.
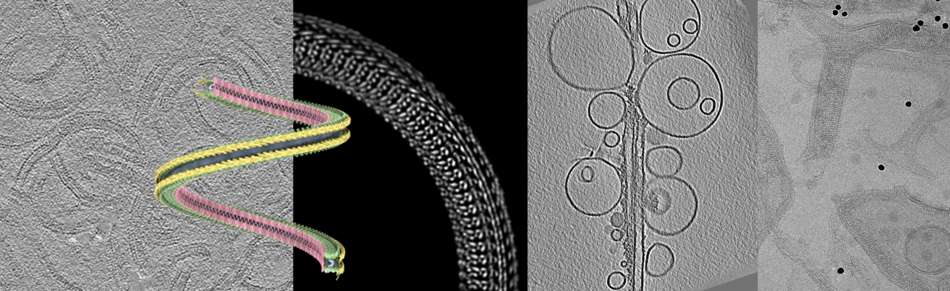
Since the ‘resolution revolution’ cryo-EM is a technique that can lead to high-resolution structures of proteins and protein complexes. In combination with tomography and subtomogram averaging, it can further reveal the architecture of larger assemblies, e.g. show how filaments interact with and shape membranes.
Cryo-EM starts with rapidly freezing the sample, embedded into a thin layer of vitreous ice on a small carrier grid. After transferring the sample into the electron microscope, images can be recorded with phase contrast, relying on the contrast between the native biological structure and the water only. As the sample ‘burns’ with increased electron radiation, the applied dose has to be kept minimal, which results in noisy images. To restore their content, averaging methods as single particle or subtomogram averaging can be applied, which produce 3-dimensional structures of the proteins. Therefore, thousands of identical particles with different orientations are aligned computationally by rotation and averaged to increase the signal-to-noise ratio.
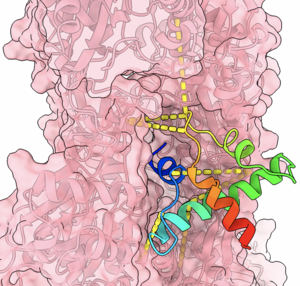
Crosslinking mass spectrometry can identify proximal protein residues in a sample and therefore provide distance restraints for protein structure modelling or identify protein-protein interactions. It is well-suited to capture transient or weak interactions, which makes it particularly suitable for the study of intrinsically disordered proteins.
To gain insights into the mechanism of nuclear reformation, we construct bottom-up models of membranes and DNA to reconstitute biological systems. By employing advanced tools such as optical tweezers, we can measure biophysical properties of our systems. Optical tweezers use highly focused laser beams to trap and move microscopic particles, such as individual molecules or cellular components, allowing us to study the forces involved in molecular interactions. We can manipulate systems at single molecular levels and measure forces with high precision. Coupled with confocal imaging, we can study how proteins bind and organize DNA and how specific molecular forces drive critical processes during nuclear reformation.

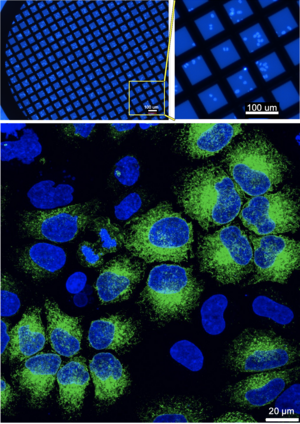
Light microscopy and electron microscopy have both individual strengths. The unmatched resolution of cryo-electron microscopy comes with the downside of lacking labels. Correlative light and cryo-electron microscopy (cryo-CLEM) combines the molecular specificity of fluorescence microscopy with the high-resolution structural detail of electron microscopy, all while preserving the sample in a near-native, frozen state. Thus, it can link structural changes to functional events.