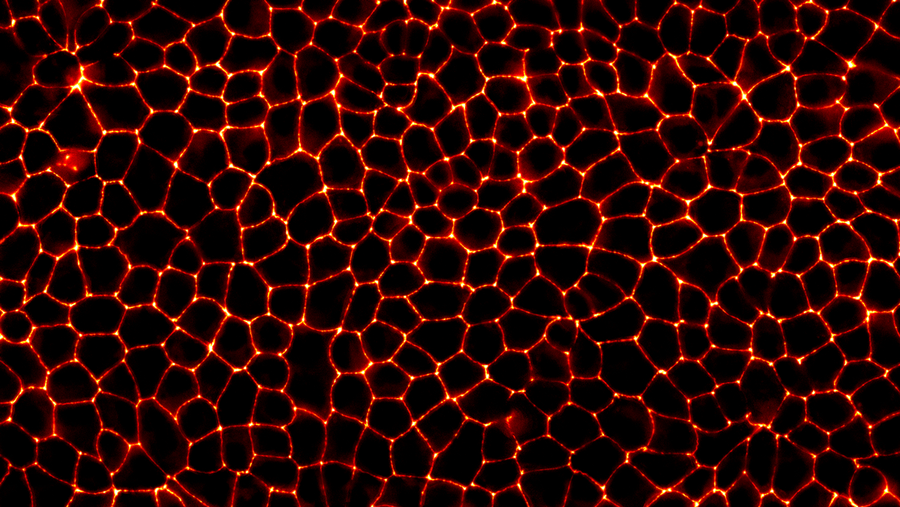
Tight junctions between the cells visualized by fluorescent microscopy © Honigmann Lab
Our bodies and organs are shielded from the external environment by tissue barriers like the skin. These barriers must be tightly sealed to prevent unwanted substances from entering. This sealing is achieved through structures called tight junctions. However, how these tight junctions form has long been a mystery. Now, an interdisciplinary team of researchers, led by Prof. Alf Honigmann at the Biotechnology Center (BIOTEC) of Dresden University of Technology and former research group leader at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG), has uncovered that the proteins responsible for these seals form a liquid-like material on the cell surface much like the water that condenses on a cold window. Their findings were published in the journal Nature.
Our skin acts as a protective shield against the outside world, and like a well-built brick wall, it must be tightly sealed to prevent breaches. Similarly, our organs like lungs or intestines must be sealed to make sure that the contents are not spilling to other body compartments. The outermost layer of our organs achieves this with specialized seals between the cells known as tight junctions.
Tight junctions are much like a joint between floor or wall tiles. They are belts that surround the top of each cell and attach to the neighboring cells to form a tight seal between them.
“Unlike the joint between the tiles or mortar in the brick wall, tight junctions are dynamic. Our skin or organs are soft and the cells change their shape constantly. Tight junctions must be able to adapt to cell shape change and still be able to seal the gaps,” explains Prof. Honigmann, chair of Biophysics and research group leader at the BIOTEC. “How tight junctions are able to form such a robust yet flexible material around the cell perimeter was an intriguing scientific question.”
Condensation on a Surface
To understand how these seals form, Prof. Honigmann’s team used advanced biophysical methods to observe the process in real-time. They developed a way to chemically switch the formation of tight junctions on and off at their will. They also used genetic engineering to tag the sealing proteins with a fluorescent marker. Together, this allowed them to use high-resolution microscopy to watch tight junctions form in real-time.
Working together with theoretical physicists led by Frank Jülicher at the Max Planck Institute for Physics of Complex Systems (MPI-PKS) in Dresden, the group was able to show that tight junction self-assembly is driven by a physical phenomenon called surface wetting.
“It is fascinating that these tight junction proteins behave in a very similar way to water. Putting together our observations and the theoretical physics modeling, we arrived at what is essentially the physical process of liquid condensation on a surface,” says Dr. Karina Pombo-Garcia, the researcher behind the project and now a research group leader at the Rosalind Franklin Institute in England.
Tight junction proteins bind to the surface of the cell membrane at the interface where the cells touch each other. When the number of proteins bound there reaches a certain threshold, the proteins condense into a liquid that gradually grows into a sort of drop on the cell surface. Eventually, these drops elongate and touch each other to form a uniform belt around the cells. In this way, tight junctions seal the spaces between the cells to make our skin and organs airtight.
“Perhaps everybody has seen it in winter. Tiny drops of water appear on a cold window. It’s exactly that but on a molecular scale,” adds Dr. Pombo-Garcia.
Liquids Made of Proteins
As early as 2017, the Honigmann team began to suspect that tight junction proteins might behave like liquids. “We put a lot of effort into figuring out how to measure and observe these liquid-like properties,” says Prof. Honigmann. “Fortunately, we were in the right place at the right time.”
The early work leading to this discovery was conducted at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden. Researchers at the MPI-CBG are pioneers of condensate biology, the newly discovered branch of biology that focuses on proteins forming large assemblies with liquid-like properties.
“Condensate biology is a promising field, because it bridges the gap between scales. One of the general problems in biology is to understand how structures like cell organelles form from the myriads of molecular interactions in the cytoplasm. We know now that certain biomolecules can self-organize into materials such as liquids and gels. This enables us to adapt well-understood physical concepts such as condensation and other phase transitions to describe structure formation in biology,” concludes Prof. Honigmann.
Press release originally published by the BIOTEC of TU Dresden: https://tu-dresden.de/cmcb/biotec/news-termine/news/wie-zellen-kondensation-nutzen-um-gewebe-fest-zu-versiegeln
Karina Pombo-García, Omar Adame-Arana, Cecilie Martin-Lemaitre, Frank Jülicher and Alf Honigmann: Membrane prewetting by condensates promotes tight-junction belt formation. Nature (August 2024). doi.org/10.1038/s41586-024-07726-0